The US Food and Drug Administration authorized Novavax's Covid 19 vaccine for emergency use in adolescents, reported CNN. It is the fourth coronaviru
The US Food and Drug Administration authorized Novavax’s Covid 19 vaccine for emergency use in adolescents, reported CNN. It is the fourth coronavirus vaccine available in the United States and, unlike the others, it uses a protein-based technology. The vaccine was authorized for Adults in July. With the latest emergency use authorization, it also will be available as a two-dose primary series for ages 12 to 17. In this age group, “overall, the clinical efficacy of the vaccine is around 80%,” said Silvia Taylor, Novavax’s senior vice president global corporate affairs. The vaccine has shown 90% overall efficacy in adults. Novavax announced in early July that its vaccine shows “broad” immune response to currently circulating variants, including the Omicron subvariants BA.4 and BA.5., reported CNN.
“One of the things that we believe makes our vaccine unique is that we actually see really good immune response against variants with our prototype vaccine. And so, if you think about the vaccine that we already have authorized that we’re talking about now to be authorized for adolescents, we actually see a good immune response against variants including Omicron, including BA.1 and BA.5,” Taylor said. Protein-based vaccines use a more traditional approach than mRNA vaccines, teaching the immune system to recognize little modified pieces of the virus that the vaccine is targeting. In this case, that means fragments of the coronavirus spike protein. Taylor told CNN that having a protein-based coronavirus vaccine available helps give adolescents more options. Next, Novavax plans to have trial data on using the vaccine among children younger than 12. “We expect those results in the early part of 2023,” Taylor said, as reported by CNN.

Watch against contagion
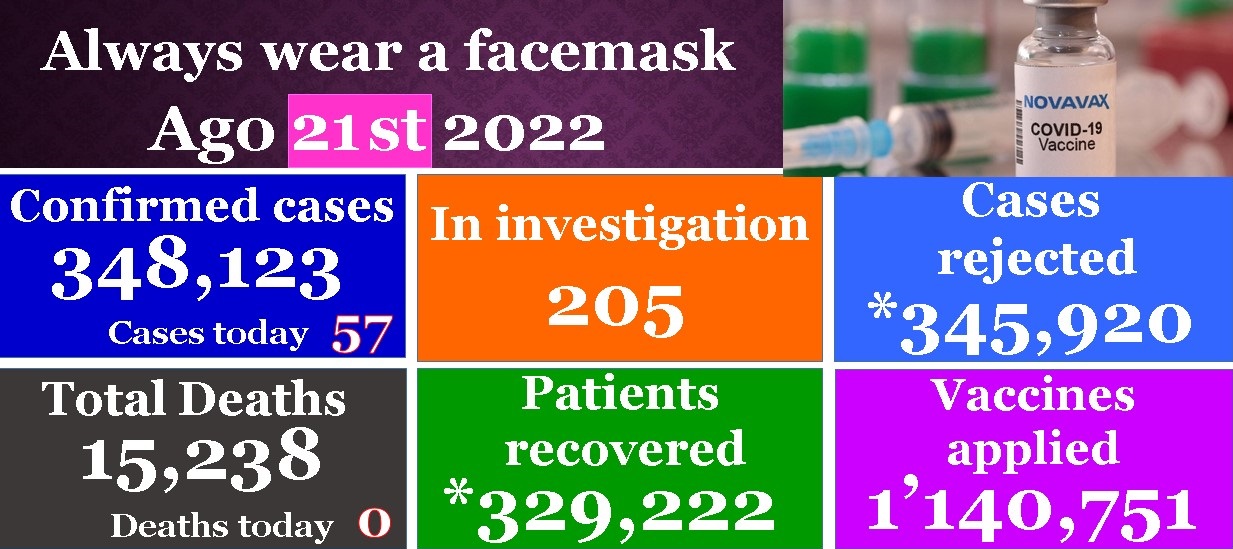
In the last 24 hours 57 new cases of contagion and 0 deaths caused by Covid 19 were reported by State and Health Authorities. Contagion is being watched in Guanajuato and cases of contagion reported are over 50 in the last 24 hours, while there were 0 deaths reported. Authorities maintain the color of activities in the GREEN easing restrictions on activities, both leisure and work.
State Health Authorities insist that Guanajuato should maintain health protection.
For more information call:
- 800 004 4800 and
- 800 627 2583
Or visit:
- https://coronavirus.guanajuato.gob.mx/
- reactivemosgto.guanajuato.gob.mx